FDA Generic Drug Approval: Complete Step-by-Step Process
Posted On December 19, 2025 11Learn the complete FDA generic drug approval process through the ANDA pathway. Understand bioequivalence requirements, submission steps, review timelines, and how generics save billions while meeting strict safety standards.

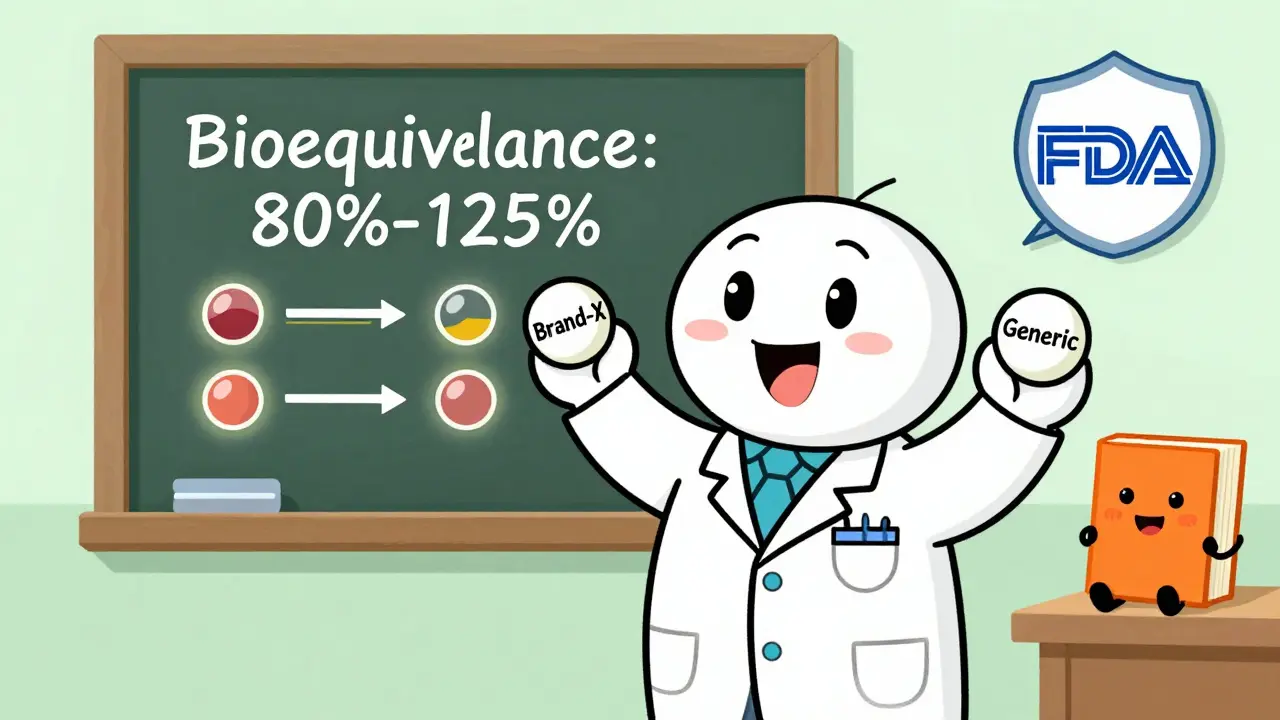




Categories