26 Jan 2026 |
Health
|
by
Brian Halstead
Antibiotics save millions of lives every year. But if you’ve ever been told, "It’s just a virus, antibiotics won’t help," you know they’re not magic pills. They’re precise tools - and using them wrong can make them useless. Understanding how different antibiotics work isn’t just for doctors. It’s for anyone who’s ever taken one, or wondered why a sore throat gets better on its own while a urinary tract infection doesn’t.
What Antibiotics Actually Do
Antibiotics don’t cure infections. They help your body do it. Bacteria grow fast. They multiply by the thousands in hours. Antibiotics slow them down or kill them outright, giving your immune system time to clean up the rest. There are two main ways they do this: bactericidal (kill bacteria) and bacteriostatic (stop them from multiplying). The difference matters - especially when your immune system is weak. The first antibiotic, penicillin, was found in 1928. It worked because it attacked something bacteria have that humans don’t: a tough outer wall. That’s still the foundation of most antibiotics today. But over 90 years later, we have dozens of classes, each with a different target, side effect, and use case.Beta-Lactams: The Original Weapon
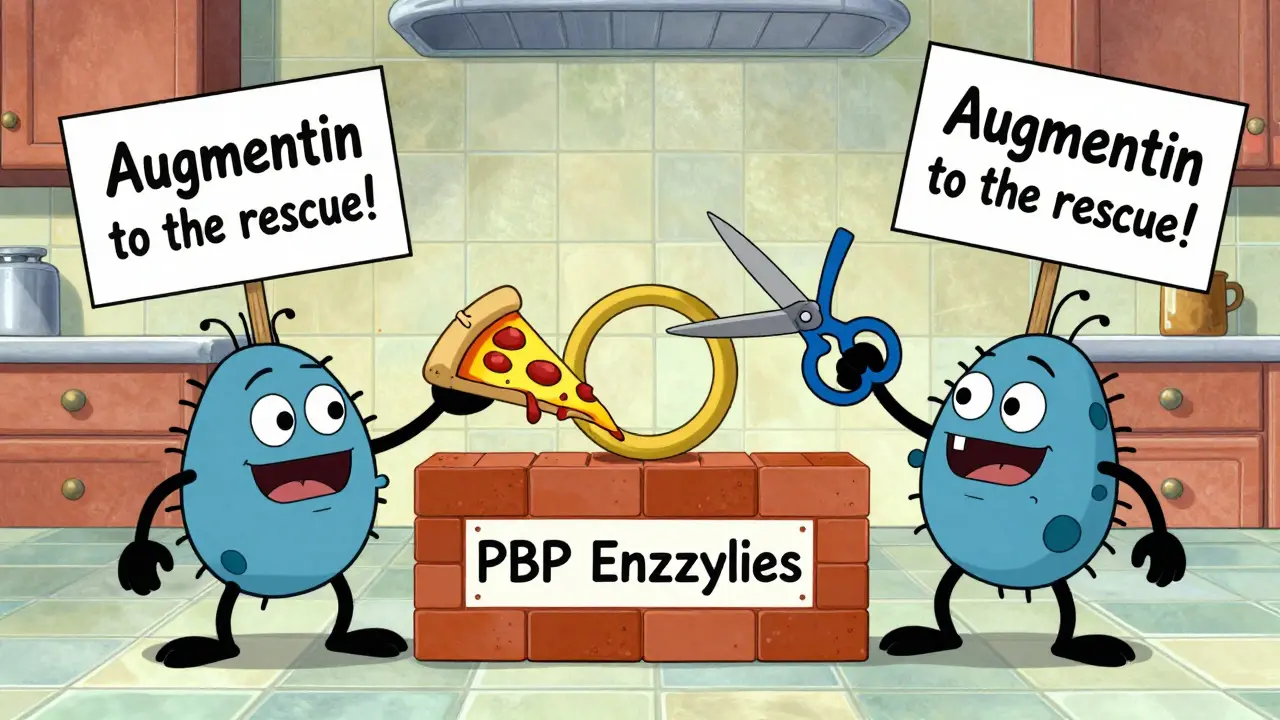
Penicillin, amoxicillin, cephalexin, ceftriaxone - these all belong to the beta-lactam family. They share a four-atom ring structure called the beta-lactam ring. This ring tricks bacteria into thinking it’s part of their own cell wall material. When bacteria try to build their wall, they grab onto this fake piece. The antibiotic then locks in place, blocking the enzymes (called PBPs) that stitch the wall together. Without a strong wall, bacteria swell up and burst from the pressure inside. It’s a clean, fast kill. That’s why beta-lactams are first-line for strep throat, ear infections, and many skin infections. But bacteria fought back. They made enzymes called beta-lactamases that chop up the ring like scissors. That’s why amoxicillin alone doesn’t work for some infections anymore. Combine it with clavulanic acid (like in Augmentin), and the enzyme is blocked. The antibiotic gets through. Cephalosporins - another beta-lactam group - come in four generations. First-gen (like cefazolin) targets mostly Gram-positive bugs like staph. Second-gen adds some Gram-negative coverage. Third-gen (ceftriaxone) hits harder Gram-negatives like E. coli and even Pseudomonas. Fourth-gen (cefepime) is broad-spectrum, used in hospitals for serious infections. Each step up adds power - and risk of side effects.Protein Synthesis Blockers: The Silent Saboteurs
Bacteria make proteins the same way we do - using ribosomes. But their ribosomes are slightly different. Antibiotics like macrolides (azithromycin, clarithromycin), tetracyclines (doxycycline), and aminoglycosides (gentamicin) slip into those bacterial ribosomes and mess up the process. Macrolides bind to the 50S part of the ribosome. They stop the protein chain from growing. That’s why they’re used for walking pneumonia, bronchitis, and some STIs. They’re bacteriostatic, so your immune system still has to finish the job. That’s fine for healthy people. Not so much for someone with a weakened system. Tetracyclines bind to the 30S subunit. They’re broad, covering everything from acne to Lyme disease. But they bind to calcium, so they stain kids’ teeth if given under age 8. They also make your skin burn in the sun. If you’re on doxycycline, skip the beach. Aminoglycosides are harsher. They bind to the 30S subunit too, but they cause the ribosome to misread genetic code. The bacteria make broken proteins - and die. They’re powerful, but toxic. They can damage kidneys and hearing. That’s why they’re reserved for serious infections like sepsis, and often used with other antibiotics. They also can’t enter anaerobic bacteria (those that live without oxygen), so they’re useless for gut infections. Linezolid, a newer oxazolidinone, blocks protein synthesis even earlier - at the very start. It’s synthetic, not natural. It’s used for resistant staph infections like MRSA. It’s expensive, and it can lower blood cell counts after long use. But when nothing else works, it’s a lifeline.
Fluoroquinolones: The DNA Destroyers
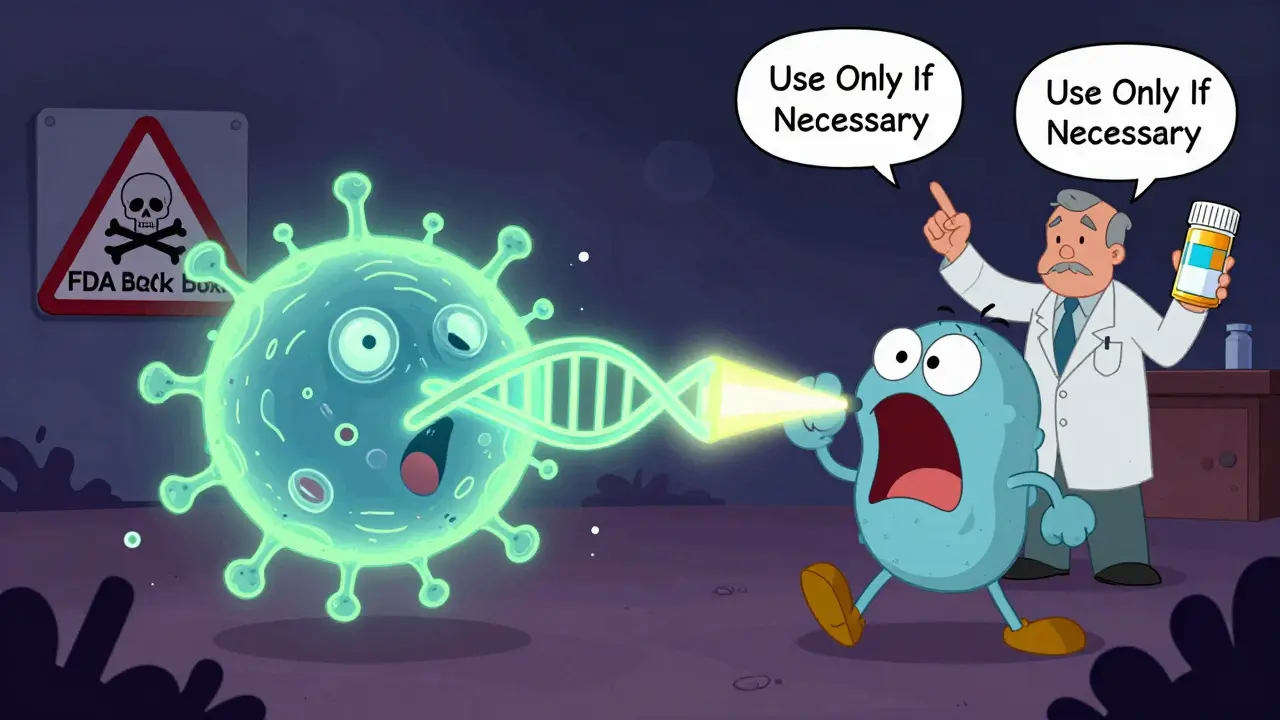
Ciprofloxacin, levofloxacin, moxifloxacin - these are fluoroquinolones. They don’t touch walls or ribosomes. They go straight for the DNA. Bacteria need to copy their DNA to multiply. Two enzymes - DNA gyrase and topoisomerase IV - untwist and separate the strands. Fluoroquinolones jam those enzymes. The DNA gets tangled. The cell can’t divide. It dies. These are powerful, broad-spectrum drugs. They get into bones, lungs, and even inside cells. That’s why they’re used for complicated UTIs, pneumonia, and some abdominal infections. But the FDA added black box warnings in 2016. They can cause tendon ruptures, nerve damage, and even mental health side effects. Because of this, they’re no longer first-choice for simple infections like sinusitis or bronchitis. Reserve them for when other antibiotics fail.Other Key Classes: Folate, Membranes, and Anaerobes
Sulfonamides (like sulfamethoxazole) block folate production. Bacteria need folate to make DNA and proteins. Humans get folate from food, so this doesn’t hurt us much. But resistance is high, so they’re rarely used alone. They still work well in combo with trimethoprim (Bactrim) for urinary infections and Pneumocystis pneumonia in immunocompromised patients. Metronidazole is the go-to for anaerobic bacteria - the kind that live in abscesses, the gut, and deep wounds. It also kills parasites like Giardia. How? It gets activated inside the bug, then shreds its DNA. But it has a nasty side effect: if you drink alcohol while taking it, you get sick. Vomiting, flushing, rapid heartbeat. That’s the disulfiram reaction. It’s not dangerous, but it’s unforgettable. Vancomycin is a glycopeptide. It binds to the end of the cell wall building blocks, preventing them from being used. It’s used for MRSA and severe C. diff infections. It’s given intravenously because it doesn’t absorb well in the gut. It can hurt kidneys and cause "red man syndrome" - a flushing reaction if infused too fast.
Why Resistance Is Growing - And What It Means
Every time you take an antibiotic, you’re playing Russian roulette with resistance. Bacteria that survive multiply. Over time, the whole population changes. The WHO says over 50% of E. coli strains in 72 countries are now resistant to fluoroquinolones. In the U.S., 30% of outpatient antibiotic prescriptions are unnecessary - for colds, coughs, viral sore throats. That’s not just waste. It’s fuel for superbugs. Worse, antibiotics disrupt your microbiome. A 2022 study showed broad-spectrum antibiotics can wipe out good gut bacteria for up to a year. That increases your risk of C. diff infection by 17 times. That’s why narrow-spectrum antibiotics - like penicillin for strep - are preferred when possible. New drugs are coming, but slowly. Cefiderocol, approved in 2019, tricks bacteria into pulling it inside by pretending to be iron. It works against carbapenem-resistant bugs. But it’s expensive. And there are only 42 new antibiotics in development globally. Only 16 target the WHO’s top-priority superbugs.Choosing the Right One
There’s no one-size-fits-all antibiotic. Doctors consider:- What infection you have (UTI? pneumonia? skin abscess?)
- Which bacteria are likely causing it
- Your allergies, kidney/liver function, age
- Local resistance patterns (e.g., is MRSA common in your area?)
- Whether you need a drug that penetrates deep tissue (like the prostate or brain)





