
When a generic drug company wants to prove their version of a medication works just like the brand-name version, they don’t test it on thousands of people. They use a smarter, leaner method called a crossover trial design. This approach isn’t just efficient-it’s the gold standard for bioequivalence studies, used in nearly 9 out of 10 cases approved by the FDA and EMA. But how exactly does it work? And why does it matter so much?
How a Crossover Trial Works
Imagine you’re testing two versions of the same pill: one made by the original drugmaker (the reference), and one by a generic company (the test). In a crossover trial, every participant takes both pills-but not at the same time. They take one first, wait a while, then take the other. Some get the generic first, then the brand; others get the brand first, then the generic. This is called a 2×2 crossover: two periods, two sequences (AB or BA). The magic here is that each person becomes their own control. Since everyone takes both drugs, differences in age, weight, metabolism, or genetics don’t skew the results. You’re not comparing John’s response to Sarah’s-you’re comparing how John responds to Drug A versus how he responds to Drug B. This cuts down on noise, letting researchers spot real differences with far fewer people. A typical study might involve just 24 healthy volunteers. That’s a fraction of what a parallel-group trial would need. In a parallel design, where one group gets Drug A and another gets Drug B, you’d need at least 72 people to get the same statistical power-if between-person differences are twice as big as measurement error. Crossover designs make that possible because they remove the variability between people entirely.The Washout Period: Why Timing Matters
You can’t just switch from one drug to another right away. If Drug A is still hanging around in your bloodstream when you take Drug B, you’ll get mixed results. That’s called a carryover effect-and it’s the biggest risk in crossover trials. To prevent this, there’s a mandatory washout period between treatments. Regulatory agencies like the FDA and EMA require it to last at least five elimination half-lives of the drug. That means if a drug clears out of the body in 8 hours, you need to wait at least 40 hours before the next dose. For drugs with longer half-lives-say, 2 weeks-you can’t use a crossover design at all. The wait would be too long, and participants would drop out. This isn’t just theory. In 2021, a bioequivalence study failed because the washout was only three half-lives long. Residual drug levels from the first period skewed the second. The company had to restart the trial using a 4-period replicate design, costing nearly $200,000 extra.Standard vs. Replicate Designs
For most drugs, the 2×2 crossover is enough. But for highly variable drugs-where the same person’s blood levels jump around a lot from dose to dose-this design doesn’t give enough data. That’s where replicate designs come in. In a replicate design, each participant takes each drug more than once. The most common versions are:- Partial replicate (TRR/RTR): One group gets Test-Reference-Reference, another gets Reference-Test-Reference. Each drug is taken twice, but not in the same order.
- Full replicate (TRTR/RTRT): Everyone gets Test-Reference-Test-Reference or the reverse. Both drugs are given twice, in alternating order.
Statistical Analysis: What Happens Behind the Scenes
It’s not enough to just give people pills and measure blood levels. The data has to be analyzed correctly. Most studies use linear mixed-effects models, often run in SAS with PROC MIXED or PROC GLM. The model checks for three things:- Sequence effect: Did the order of drugs affect the outcome? (e.g., did everyone do better on the second drug just because they were more experienced?)
- Period effect: Did time itself change the results? (e.g., seasonal changes in metabolism or diet?)
- Treatment effect: Is there a real difference between the test and reference drugs?
Why Crossover Designs Dominate
Over 89% of bioequivalence studies submitted to the FDA in 2022-2023 used crossover designs. Why? Because they’re cost-effective, ethical, and statistically powerful. A clinical trial manager in 2022 saved $287,000 and eight weeks by choosing a 2×2 crossover over a parallel design for a generic warfarin study. With an intra-subject coefficient of variation of 18%, they needed only 24 subjects. A parallel design would have required 72. CROs like PAREXEL and Charles River report that 75-80% of their bioequivalence work uses crossover methods. The global bioequivalence testing market, worth $2.87 billion in 2022, runs on this model. Regulators agree. The FDA’s guidance says a crossover design “is recommended.” The EMA calls it the “preferred design.” Even critics like Dr. Stephen Senn acknowledge that when done right, it’s the most efficient way to compare treatments.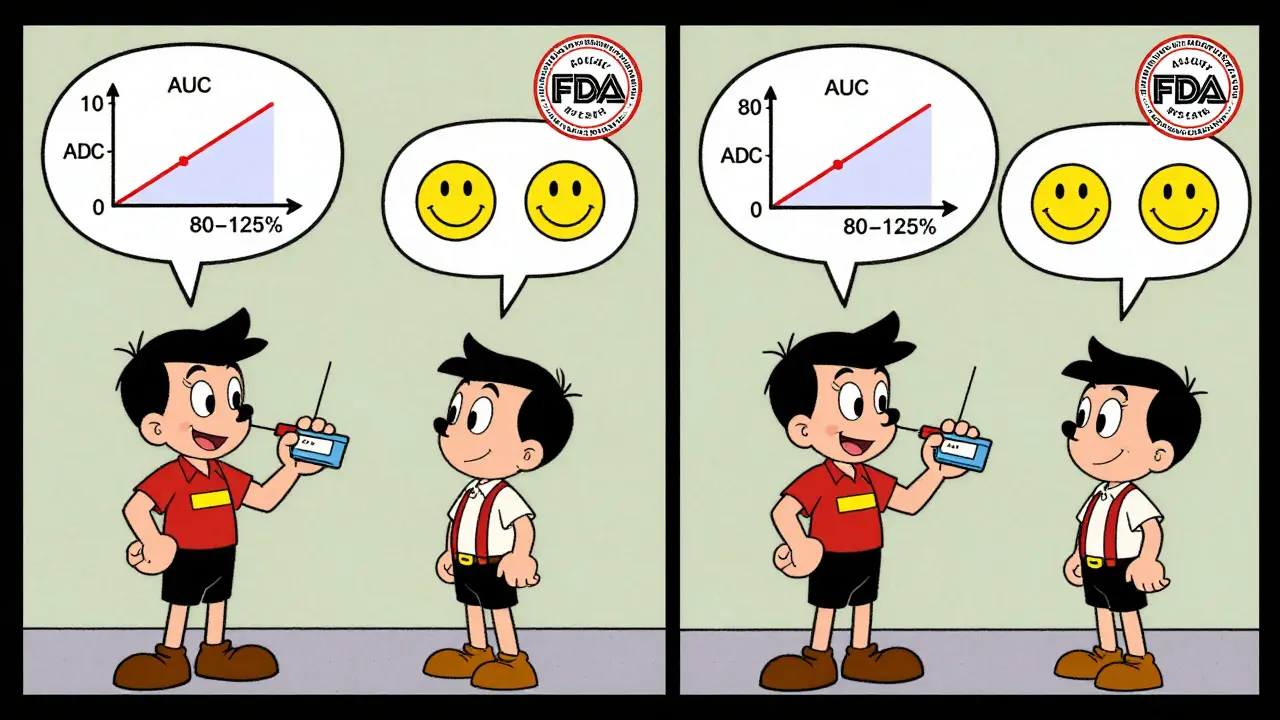
When Crossover Doesn’t Work
It’s not perfect. Crossover trials can’t be used for drugs with very long half-lives. They’re also risky for drugs with irreversible effects-like chemotherapy agents-or for conditions where the disease state changes permanently after treatment. They also require more visits, more blood draws, and longer study durations. Each participant might need to come in 6-10 times over 6-8 weeks. That’s a burden. But for most oral solid dosage forms-pills, capsules, tablets-it’s worth it. And while carryover effects are a concern, they’re manageable. Proper washout validation, sequence randomization, and statistical testing for sequence-by-treatment interaction can catch problems before they ruin a study.The Future of Crossover Designs
The field is evolving. The FDA’s 2023 draft guidance now allows 3-period replicate designs for narrow therapeutic index drugs-like warfarin or levothyroxine-where even small differences can be dangerous. The EMA is expected to update its guidelines in late 2024 to formally recommend full replicate designs for all highly variable drugs. And adaptive designs-where sample size is adjusted mid-study based on early data-are growing. In 2022, 23% of FDA submissions included adaptive elements, up from 8% in 2018. Some experts predict that wearable sensors and continuous blood monitoring could one day eliminate the need for washout periods altogether. Imagine tracking drug levels in real time-no more waiting five half-lives. But for now, the crossover design remains the backbone of bioequivalence testing.What You Need to Remember
- Crossover trials compare the same person on two treatments, reducing variability and cutting sample sizes by up to 80%.
- The standard design is 2×2 (AB/BA) with a washout period of at least five half-lives.
- For highly variable drugs, use replicate designs (TRR/RTR or TRTR/RTRT) to enable reference-scaled bioequivalence.
- Statistical analysis must test for sequence, period, and treatment effects-and exclude any participant with missing data.
- 90% confidence intervals for AUC and Cmax must fall within 80-125% (or 75-133% for highly variable drugs under RSABE).
- Improper washout periods are the #1 reason studies get rejected by regulators.
If you’re developing a generic drug, choosing the right crossover design isn’t just a statistical decision-it’s a regulatory necessity. Get it wrong, and you lose time, money, and market access. Get it right, and you bring an affordable medicine to patients faster.
What is the main advantage of a crossover design in bioequivalence studies?
The main advantage is that each participant serves as their own control, eliminating variability between individuals. This allows researchers to detect true differences between drugs with far fewer people-often six times fewer-than a parallel-group study. It’s more efficient, ethical, and cost-effective.
Why is a washout period necessary in a crossover trial?
A washout period ensures the first drug is completely cleared from the body before the second drug is given. If traces remain, they can interfere with the second treatment’s results, creating a carryover effect. Regulatory guidelines require at least five elimination half-lives between doses to prevent this.
When should a replicate crossover design be used instead of a standard 2×2 design?
A replicate design is used for highly variable drugs-those with an intra-subject coefficient of variation above 30%. These drugs show large fluctuations in blood levels even when the same person takes the same dose repeatedly. Replicate designs allow regulators to use reference-scaled bioequivalence (RSABE), which adjusts the acceptance range based on the drug’s natural variability.
What are the regulatory acceptance criteria for bioequivalence?
For most drugs, the 90% confidence interval of the test-to-reference ratio for AUC and Cmax must fall between 80.00% and 125.00%. For highly variable drugs, regulators may allow a wider range of 75.00% to 133.33% using reference-scaled average bioequivalence (RSABE), but only if the reference drug’s variability justifies it.
Can crossover designs be used for all types of medications?
No. Crossover designs are unsuitable for drugs with very long half-lives (e.g., over two weeks), where the required washout period would be impractical. They’re also avoided for drugs with irreversible effects, such as chemotherapy agents, or for conditions where treatment permanently alters the disease state.





